OAN Brooke Mallory
UPDATED 6:11 PM – Wednesday, April 19, 2023
The U.S. Food and Drug Administration (FDA) is now claiming that in order to make the immunization process simpler for the majority of people, the emergency use authorizations (EUAs) for the original Moderna and Pfizer-BioNTech COVID-19 mRNA vaccines are no longer in effect.
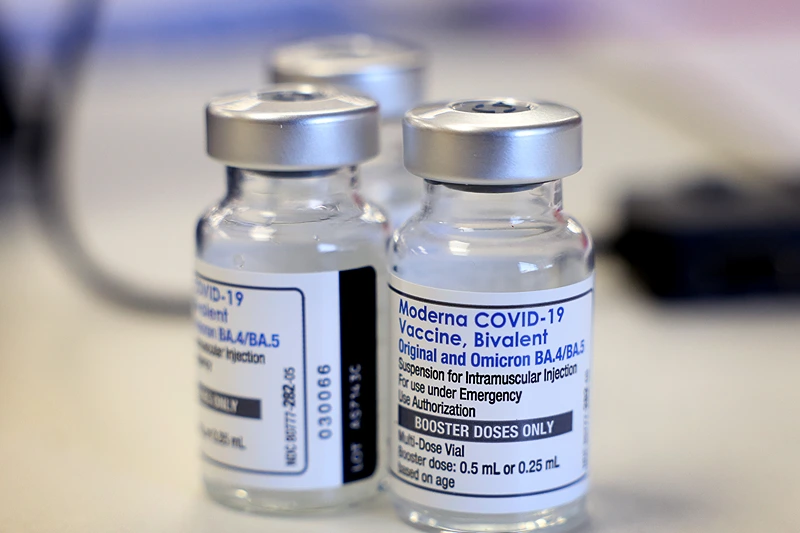
However, the existing bivalent vaccinations (original and omicron BA.4/BA.5 strains) are now authorized to be used for all doses given to people six months of age and older, including an additional dosage or doses for specific high-risk demographics.
“We do have vaccines that are available to protect against these severe consequences, so why not use them? They don’t do any good just sitting on a shelf. So, let’s give them to individuals who are at the highest risk who can benefit the most,” said Dr. Matthew Laurens, who works at the University of Maryland School of Medicine.
Advertisement
The organization added that any adult who has not yet received a dose of the updated bivalent vaccine will do so, instead of starting with the original vaccines and receiving the updated bivalent shot as a booster.
The original doses of the Moderna and Pfizer vaccines were created, evaluated, and authorized for emergency use in under a year. They were initially said to be almost 100% effective, even against new variants. Major studies frequently asserted that they were extremely effective against COVID-19 as late as 2021.
The public was consistently persuaded by the FDA, the Center for Disease Control and Prevention (CDC), Dr. Anthony Fauci, and mainstream media outlets that the original series of vaccines would offer guaranteed beneficial, long-lasting protection.
The FDA subsequently distributed the vaccines with complete approval, despite growing evidence of their fast-declining effectiveness against the virus.
Based on expert claims and FDA permission, the Biden administration then promoted illegitimate vaccine mandates, which jeopardized Americans and their careers at different companies all across the country if they refused to take a COVID-19 vaccine. Many people who refused to receive a COVID-19 vaccine lost their job, or even lost friends or relatives who were fearful and convinced by media outlets into believing that the shots were a life-or-death predicament.
It was later discovered that COVID-vaccinated and boosted individuals were still becoming sick and testing positive for the virus.
“At this stage of the pandemic, data support simplifying the use of the authorized mRNA bivalent COVID-19 vaccines and the agency believes that this approach will help encourage future vaccination,” said Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research.
The first strain of the virus, which was first discovered in Wuhan, China, was matched with the first COVID-19 vaccine. The omicron variation and its subvariants were believed to be the most common strains worldwide, but that strain of the virus is no longer in circulation, according to the FDA.
In the U.S., vaccine uptake has really slowed down. Data from the CDC shows that just 16.7% of Americans and less than half of those over 65 have received a bivalent booster vaccine. The FDA will be allowing seniors over 65 years old to receive a second bivalent booster shot, although it is still unclear how frequently this will occur.
“Simple and clear messaging is critical when it comes to ensuring vaccine uptake… Moving to a model that resembles the yearly flu shot campaign will help focus messaging and help increase population immunity ahead of a potential fall surge,” said Dr. John Brownstein, an epidemiologist and chief innovation officer at Boston Children’s Hospital.
In order to explore the idea of modifying the vaccine formulation for the fall of 2023, FDA advisors will meet sometime in June.
In addition to the FDA authorization modifications affecting the mRNA vaccines made by Pfizer and Moderna, Novavax confirmed that its protein-based COVID-19 vaccine, which has a different formulation, is still offered in the United States.
Adults who have already received two doses of the vaccine may receive a third dose as long as they are at least 18 years or older.
“COVID-19 is not influenza… But that said, we’re using that public health model where we’ll look to do our best to select what we believe to be likely to circulate the following fall and winter season, and use that in the vaccine composition to try to protect as many people during the season in which respiratory viruses tend to wreak havoc.”
Despite these measures, studies on COVID-19 are still underway, and experts are reportedly trying to comprehend the seasonal patterns and viral development. According to Brownstein, the revised COVID-19 immunization strategy might not be the last adjustment.
“We still have to recognize that the future of COVID remains uncertain and this trial vaccination strategy may have to be modified,” Brownstein said.
The FDA stated in the press release that, “Available data show that almost all of the U.S. population 5 years of age and older now have antibodies as a result of either vaccination or infection against SARS-CoV-2. The use of bivalent COVID-19 vaccines for all doses administered to individuals 6 months of age and older is supported by the data described below, as well as post-marketing data, including real-world data, with the monovalent and bivalent mRNA COVID-19 vaccines, which have been administered to millions of people, including young children. A second bivalent dose for individuals 65 years of age and older is supported by data showing the waning of immunity in this population over time and its restoration by an additional dose. Additionally, based on evidence from studies conducted previously, immunocompromised individuals may require additional doses.
Stay informed! Receive breaking news blasts directly to your inbox for free. Subscribe here. https://www.oann.com/alerts

